Mechanical Forces Can Contribute to Gene Expression During Development
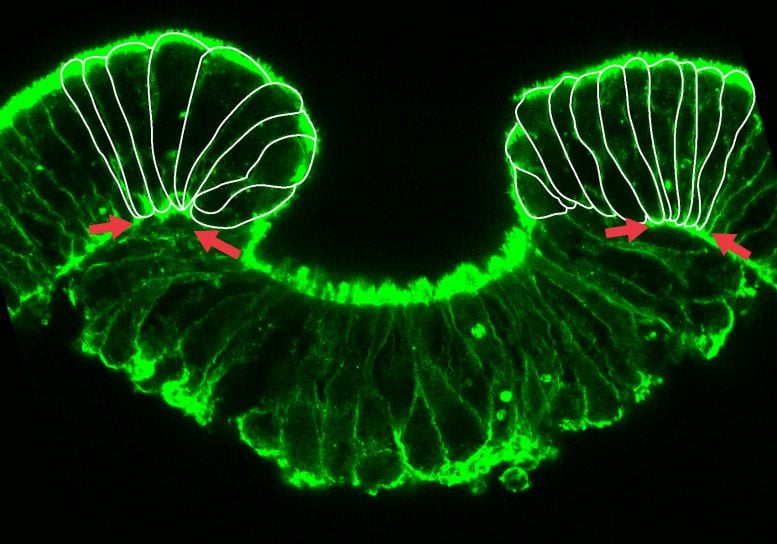
Early
stage of invagination of the inner cell layer, the endoderm. Cells at
the margins show a strong deformation (Copyright: Ulrich Technau).
It is generally thought that embryonic development and cellular differentiation of animals and humans follows a precise genetic program of spatiotemporal gene expression. However, a number of recent studies suggested that mechanotransduction – the ability of cells to transform mechanical forces into biochemical signals – can also contribute to the regulation of gene expression and thus may play an important role in development. While most of these studies were done in cell culture, the team of Ulrich Technau from the University of Vienna now reports experiments with mechanosensitive gene expression during early development of the starlet sea anemone Nematostella vectensis.
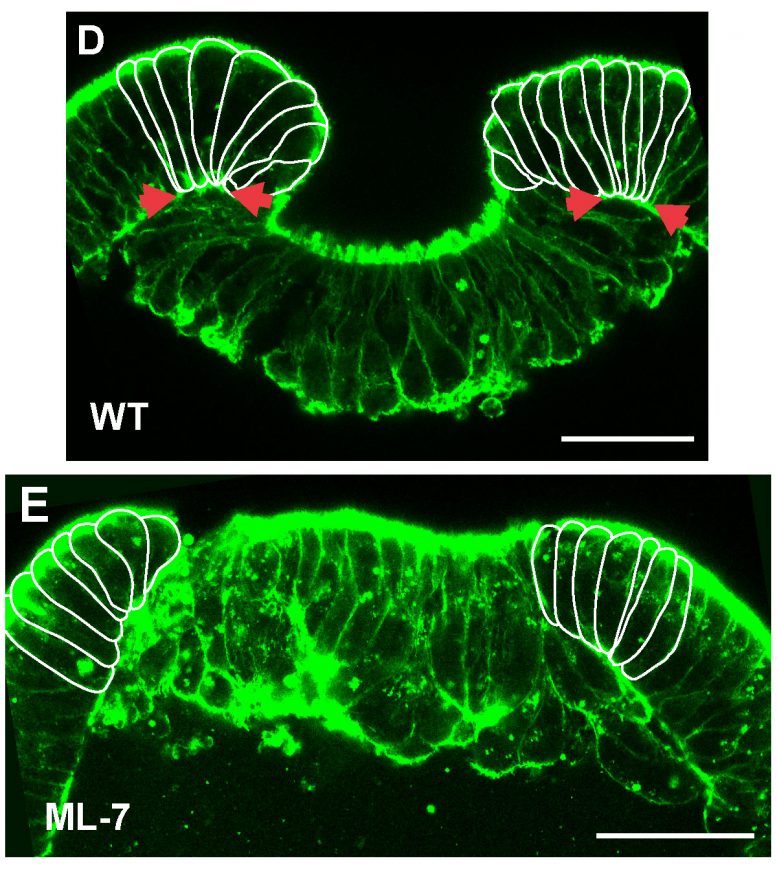
In
embryos treated with the myosin inhibitor ML-7, cells at the margin do
not deform and invagination is blocked (Copyright: Ulrich Technau).
Based on their findings, the authors propose a feedback loop whereby mechanical and genetic regulation work together to ensure robust brachyury expression. In addition, because β-catenin-dependent mechanotransduction occurs in other animals like zebrafish and the fruitfly, the findings suggest that this form of gene regulation dates back to at least 600 million years ago, the evolutionary split between vertebrates, insects and sea anemones.
Publication: katerina Pukhlyakova, et al., “β-Catenin–dependent mechanotransduction dates back to the common ancestor of Cnidaria and Bilateria,” PNAS, 2018; DOI: 10.1073/pnas.1713682115


Your Opinion is valid .